Melalite Cream (Generic Aclaro) - Active Ingredient And Chemical structure
The active ingredient contained in Melalite Cream is Hydroquinone USP 4% . Hydroquinone occurs as fine white needles. This medication is freely soluble in water and alcohol. The structure is shown below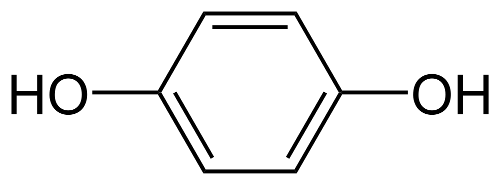
Generic Forms and Brand names of Aclaro
Melalite Cream manufactured by Abbott India Ltd., is an effective treatment to lighten areas of darkened skin such as freckles, age spots, chloasma, and melasma (dark skin discoloration).
Aclaro which has the active ingredient Hydroquinone is also sold as Hydroquinone Topical Cream, Alera, Alphaquin HP, Alustra, Claripel, Eldopaque, Eldoquin, Eldoquin Forte, EpiQuin Micro, Esoterica, Glyquin, Glyquin-XM, Hydroquinone cream, Lustra, Melpaque HP, Melquin HP, Melquin-3, Nuquin HP, Solaquin, Melanex, Melanol, Viquin Forte, Esoterica Sensitive Skin, Nava-SC, Remergent HQ, EpiQuin Micro Pump and under various other brand names.
Melalite Cream (Generic Aclaro) - Uses
Melalite Cream (Hydroquinone Topical Cream) in the form of Melalite Cream is used for the gradual treatment of ultraviolet induced dyschromia and discolation resulting from the use of oral contraceptives, pregnancy, hormone replacement therapy or skin trauma.
Melalite Cream - Preparations
Melalite Cream is available as tubes of 30 grams of cream. The strength is 4 %w/w.
Melalite Cream - Contraindications
Melalite Cream is contraindicated in persons that have a hypersensitivity or allergic reaction to Hydroquinone or any inactive ingredient present in the medication.
Melalite Cream - Warnings
The active ingredient present in Melalite Cream is a depigmenting agent which may produce unwanted cosmetic effects if not used as directed. Skin sensitivity has to be tested before using Melalite Cream by appling a small amount to an unbroken patch of skin and checking it within 24 hours. In case there is itching, vescile formation, or exessive inflammatory response further treatment is not advised. Contact with eyes should be avoided. If no lightening effect is observed after two months of treatment use of Melalite cream should be avoided.
Melalite Cream (Generic Aclaro) - Storage Requirements
Melalite Cream (Generic Aclaro) are to be stored at room temperature (15°C to 30°C). Store away from heat, moisture, and light. Keep away from children.
Melalite Cream (Generic Aclaro) - Dosage
Melalite Cream should be applied to the affected areas twice daily or as directed by your doctor.
Side Effects Of Melalite Cream (Generic Aclaro)
The most common side effects of Melalite Cream (Generic Aclaro) are cutaneous hypersensitivity (localised contact dermatitis) in which case treatment should be stopped and the doctor should be notified immediately. No systemic reactions have been reported.

















