Ibugesic Tablets (Generic Motrin) - Active Ingredient And Chemical structure
The active ingredient contained in Ibugesic Tablets is Ibuprofen. Ibuprofen possesses analgesic, antipyretic and anti-inflammatory properties, similar to other non-steroidal anti-inflammatory drugs (NSAIDs). The structure is shown below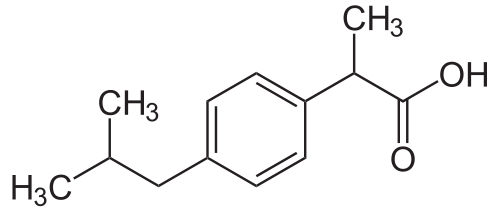
Ibugesic Tablets (Generic Motrin) - Uses
Generic Motrin (Ibuprofen) in the form of Ibugesic Tablets is used to treat Rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, cervical spondylosis, Low back pain, fibrositis, sciatica, Toothaches, relief of mild to moderate pin associated with oral surgical procedures and dental extractions as well as Primary Dysmenorrhoea.
Generic Forms and Brand names of Ibuprofen
Ibugesic Tablets manufactured by Cipla Ltd. , India is an effective treatment for arthritis, fever, as an analgesic (pain reliever), especially where there is an inflammatory component, and dysmenorrhea. Generic Motrin which has the active ingredient Ibuprofen is also sold as Advil, Brufen, Calprofen, Ribafen, Nurofen and under various other brand names.
Ibugesic Tablets (Ibuprofen ) - Preparations
Ibugesic Tablets is available as tablets of 200 mg, 400 mg and 600 mg . Each film coated tablet of Ibugesic contains Ibuprofen IP 200 mg, 400 mg or 600 mg respectively.
Ibugesic Tablets (Generic Motrin) - Storage Requirements
Ibugesic Tablets are to be stored at room temperature (15°C to 30°C). Store away from heat, moisture, and light.
Ibugesic Tablets (Generic Motrin) - Dosage
The usual adult daily dose for Ibugesic Tablets is 1.2 to 1.8 gm daily in divided doses. This can be increased to 2.4 gm total daily dose, if necessary, in acute conditions. Ibugesic Tablets (Ibuprofen) should not be used for more than a few days at a time except on medical advice.
Ibugesic Tablets (Generic Motrin) - Contraindications
Ibuprofen is contraindicated for use in patients with a known hypersensitivity to ibuprofen or any of the other ingredients present in the medicine. It is also contraindicated in persons with a known hypersensitivity to aspirin and other NSAIDs, asthma that is aspirin or NSAID sensitive or in cases where the patient has active gastrointestinal bleeding or peptic ulcera.
Side Effects Of Ibugesic Tablets (Ibuprofen)
Adverse effects with over the counter or short-term use Ibuprofen are rare. The most common side effects of Ibugesic Tablets (Generic Motrin) may include the following:
Gastrointestinal - dyspepsia, heartburn, nausea, loss of appetite, stomach pain, diarrhoea
Central nervous system (CNS) - dizziness, fatigue, headache, nervousness
Hypersensitivity reactions - skin rashes and itching. Rarely exfoliative dermatitis and epidermal necrolysis have been reported with ibuprofen. Rare cases of photosensitivity
Cardiovascular - fluid retention and in some cases oedema.